'Don't you work with old people?' Many elder-care workers still refuse to get COVID-19 vaccine
ProPublica is a Pulitzer Prize-winning investigative newsroom. Sign up for The Big Story newsletter to receive stories like this one in your inbox.
They are two sisters in two states. Both are dedicated health care professionals who watched in horror as COVID-19 swept through the nation's nursing homes, killing a staggering number of residents and staff alike.
One sister is now vaccinated. The other is not.
“ Dude. Get vaccinated!" Heidi Lucas texted her sister Ashley in May from her home in Jefferson City, Missouri.
“Nope lol," Ashley Lucas texted back from Orbisonia, Pennsylvania.
“Don't you work with old people?"
“Yeah"
“What if you killed one of them? Get vaccinated," Heidi wrote.
Neither sister is budging as the Delta variant brings a new spike in coronavirus numbers across the nation.
Their divide mirrors America's larger one, where the vaccine to combat COVID-19 is eagerly embraced by some, yet eyed with suspicion and rejected by others.
It is the refusal group, including a significant percentage who work in the nation's nursing homes, that has confounded and alarmed health care officials who are at a loss as to how to sway them.
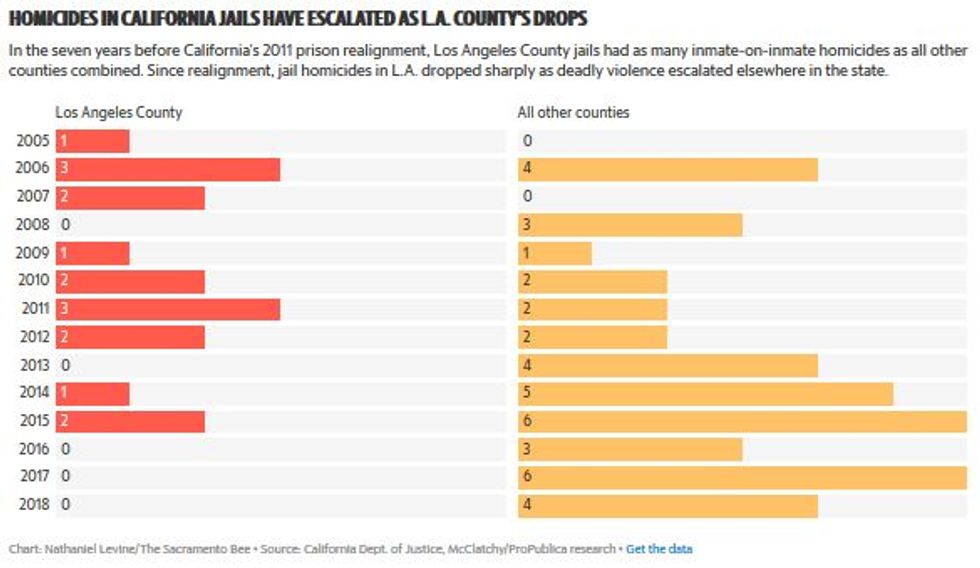
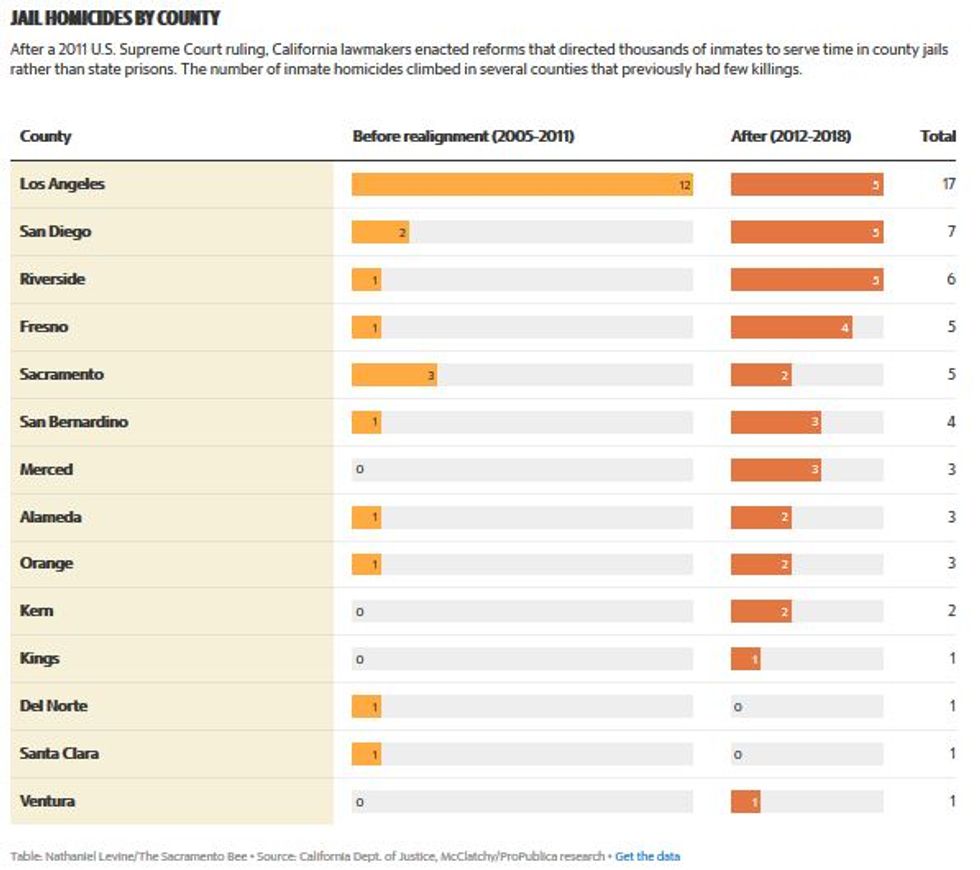
Nursing homes faced a shocking mortality rate during the pandemic. In the U.S., COVID-19 killed more than 133,000 residents and nearly 2,000 staff members between May 31, 2020 and this July 4, according to Centers for Medicare & Medicaid Services reports. The true toll is thought to be even higher as data gathering lagged in the early months of the crisis, health experts say.
Working in a nursing home became one of the “most dangerous jobs" in America in 2020, according to an analysis of work-related deaths by Scientific American.
Yet seven months after the first vaccines became available to medical professionals, only 59% of staff at the nation's nursing homes and other long-term care facilities are fully or partially vaccinated — with eight states reporting an average rate of less than half, according to CMS data updated last week.
Twenty-three individual facilities had vaccination rates of under 1%, the data showed.
Staff vaccinations have lagged even as the overall rate for residents climbed to 83%, according to the CMS data.
The strong vaccination percentage among nursing home residents is credited, in part, to an early campaign to bring the vaccine directly to facilities. That suggests availability is not necessarily the issue behind staff going without.
So, what is?
The question defies easy answers. Vaccine refusal is regional and often aligns not only with individuals' political alignment but also with their preferred news sources and which social media they follow.
Last week, President Joe Biden took aim at Facebook and other social media giants for failing to police vaccine misinformation that amplifies conspiracy theories and discourages people from getting vaccinated. “They're killing people," he said, directly blaming the platforms. On Monday, he recast the accusation to say it was specific individuals posting dangerous information who are culpable.
On Tuesday, U.S. Sen. Mitch McConnell, R-Ky., pleaded to “anyone out there willing to listen: Get vaccinated." While not mentioning skeptics specifically — including those in his own party — the Republican leader urged the unvaccinated to ignore “demonstrably bad advice."
COVID-19 cases are now surging in every state, with new hospitalizations and deaths almost entirely occurring among the unvaccinated. “This is becoming a pandemic of the unvaccinated," Centers for Disease Control and Prevention Director Rochelle Walensky warned last week during a White House briefing.
In May, CMS began requiring weekly reports on vaccinations of residents and staff at nursing homes and other long-term care facilities. The emerging data confirms many health care experts' worst fears, especially for Southern states.
Louisiana has the lowest statewide average: Just 44.5% of the staff at its long-term care facilities have been at least partially vaccinated, according to CMS data released last week.
Florida, the second lowest-vaccinated state, had a rate of just under 46% among its nursing home and long-term care staff, with Missouri, Oklahoma, Tennessee, Georgia, Mississippi and Wyoming all showing rates of less than 50 percent, according to the data.
Vaccination rates in assisted living facilities are not included in the data.
A separate American Association of Retired Persons analysis, released last week, showed that only one in five of the nation's more than 15,000 nursing homes were able to hit a goal, set by two industry trade groups, of vaccinating 75% of their staff by the end of June.
While cases in nursing homes have recently slowed, and most of the new COVID-19 infections are among younger people, some experts still worry of a return to darker days.
The CDC recently launched an investigation into deaths of residents at several western Colorado senior facilities possibly linked to unvaccinated staff, the Associated Press reported Wednesday.
“We need to sound the alarm," said Susan Reinhard, senior vice president of AARP and director of its Public Policy Institute. “Nursing homes were devastated by COVID-19, and many residents remain highly vulnerable to the virus."
Nationally, more than 89% of people 65 or older have received at least partial vaccination, the CDC reported this week. Still, public health experts have warned that even if fully vaccinated, the elderly may be vulnerable to “breakthrough" coronavirus infection because of compromised immune systems and other underlying health problems.
In Missouri's southern region, the overall rate of full vaccination in some rural counties is less than 20%, according to state health department and CDC tracking. The latest surge of the delta variant has turned the area into a “tinderbox," Steven Edwards, CEO of the CoxHealth hospital system in Springfield, recently told reporters.
On Thursday, 160 patients were being treated for COVID-19 at CoxHealth, a spokesperson told ProPublica. On May 14, there were 18.
Heidi Lucas directs the Missouri Nurses Association. She is pro-vaccine and has been pushing hard for nurses to get vaccinated, especially those on the front lines of patient care.
Lucas said it is impossible to separate the lack of vaccination among staff from the lack of vaccinations in individual communities. “Nurses are people too," she said. “They are on social media and are inundated with false information. How do you fight it?"
Her sister, Ashley Lucas, lives 900 miles away in Orbisonia, a small town of around 500 people about an hour south of State College. She's a traveling certified nursing assistant at area nursing homes and chose to skip the vaccine.
Her fiance and her children, ages 12 and 13, are also unvaccinated. “I don't consider myself an anti-vaxxer," she told ProPublica, bristling that some might see her as reckless or ill-informed.
Instead, she said her decision was carefully considered. It never made sense to her, she said, that the virus seemed to strike randomly, with some residents getting sick while others did not. She said she is not convinced the vaccine would change the odds.
She's also concerned after hearing that the vaccine could interfere with fertility — a contention that has been deemed false by the Centers for Disease Control and Prevention and the World Health Organization. It all leads her to believe more research is needed into the vaccines' long-term effects.
“This is just a personal choice and I feel it should be a free choice," she said. “I think it's been forced on us way too much."
Certified nursing assistants make up the largest group of employees working in nursing homes and other long-term care facilities, providing roughly 90 percent of direct patient care. They are typically overworked and underpaid, most earning about $13 per hour and receiving no paid sick leave or other benefits, said Lori Porter, co-founder and CEO of the National Association of Health Care Assistants.
Porter said she is not completely surprised by the low vaccination rate. It comes down to trust, she said, both of the vaccines and of facility administrators who now say staff must get vaccinated. Refusal may feel like empowerment. “It's the first time ever they have had the ball in their court," Porter said.
On March 31, Houston Methodist Hospital mandated that all of its 26,000 employees be vaccinated by June 7 or lose their jobs. Jennifer Bridges, a nurse, sued along with 116 other employees, claiming the health care system had overstepped its rights and that she and the others refused to be “human guinea pigs," evoking the Nuremberg Code, a set of ethical standards established in response to Nazi medical experimentation in concentration camps.
On June 12, U.S. District Judge Lynn N. Hughes dismissed the closely watched case, taking offense to likening the vaccine to the Holocaust, which he called “reprehensible." Ten days later, 153 Houston Methodist employees either were fired or quit after refusing the vaccine. The judge's ruling has been appealed.
A handful of long-term care chains have similarly sought to mandate worker vaccines, but such action is far from widespread in the industry. One sticking point has been whether vaccination can legally be required, since all three available vaccines have only emergency use authorization, not full approval from the U.S. Food and Drug Administration.
The thornier issue, though, is whether the facilities can risk losing staff when they're already short-handed. Many workers have vowed to quit rather than be forced into vaccinations.
Aegis Living, a long-term senior care provider in three Western states, made vaccines mandatory for its roughly 2,600 employees on July 1. Dwayne Clark, founder and CEO, said initially 400 employees refused but when the deadline arrived, only about 100 left rather than be vaccinated.
“We lost some staff that we didn't want to lose," Clark told ProPublica, “but it felt like the right moral protocol to impose."
Recently the U.S. Equal Employment Opportunity Commission issued guidelines stating that employers can require workers to be vaccinated as long as medical or religious exemptions are permitted.
“Nursing home workers certainly have the right to make decisions about their own health and welfare, but they don't have the right to place vulnerable residents at risk," said Lawrence Gostin, a health law professor at Georgetown University. “Nursing homes don't just have the power to require vaccinations, they have the duty."
Still, the issue is far from resolved.
“America is a highly litigious country," Gostin said, “I expect the courts to consistently uphold nursing home mandates, because they are entirely lawful and justified. But there will likely be lawsuits at least until it is quite clear they are futile."
Diane Peters is a registered nurse in the Chicago suburbs who last year worked at a nursing home and is now working at a senior rehabilitation center. She does not trust the science behind the vaccine and is unvaccinated. So is her fiance.
Everything about the rollout felt like propaganda, she said. Development was too rushed. Clinical trials typically take years, she said, not months. “I don't think it's safe right now, it needs more time," she said she tells patients if they ask.
Most don't, she said. Neither do her co-workers. She has only been asked once by her employer if she was vaccinated, she said, declining to name the company.
Peters guesses about 40 percent of her colleagues are also unvaccinated, but said no one likes to talk about it because the divide surrounding the vaccine is “surreal." Staff members are tested regularly and are required to wear masks, she said.
She is doubtful mandates would stick. “They can threaten," she said, “but a lot of nurses would walk."
She trusts her instincts and her own research for now. When asked what would change her mind, she had one word: “Nothing."
- The New York Times profiles 'most influential' purveyor of online ... ›
- COVID vaccine opposition is on the rise among married couples ... ›
- GOP lawmakers see widening divide on COVID vaccine as case ... ›